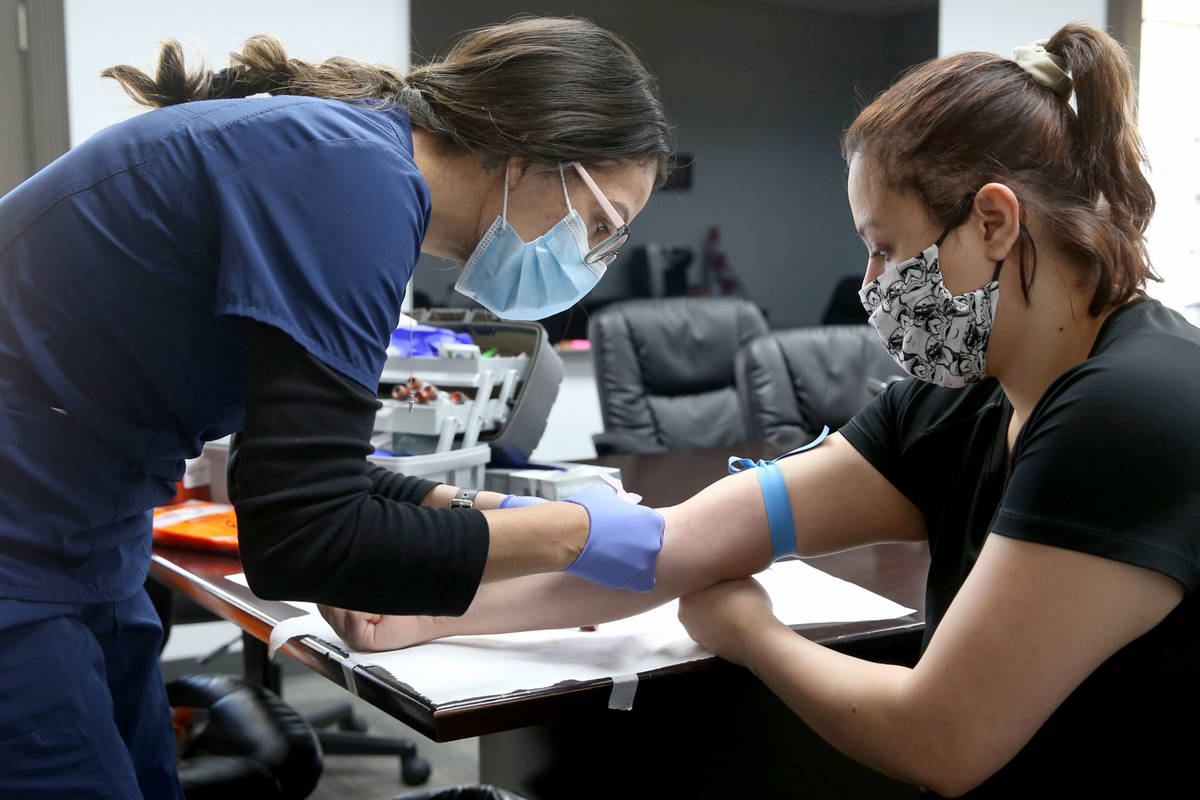
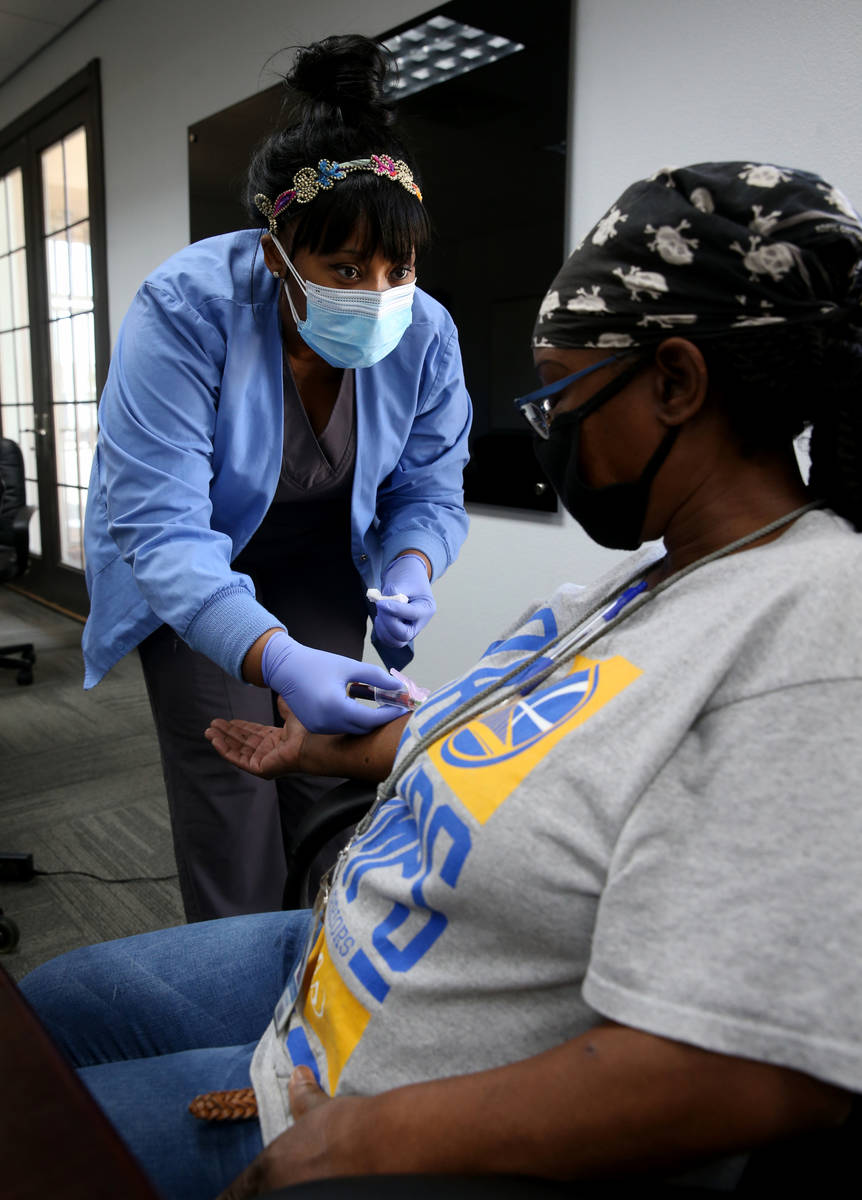
Employer offers Henderson workers free coronavirus antibody tests
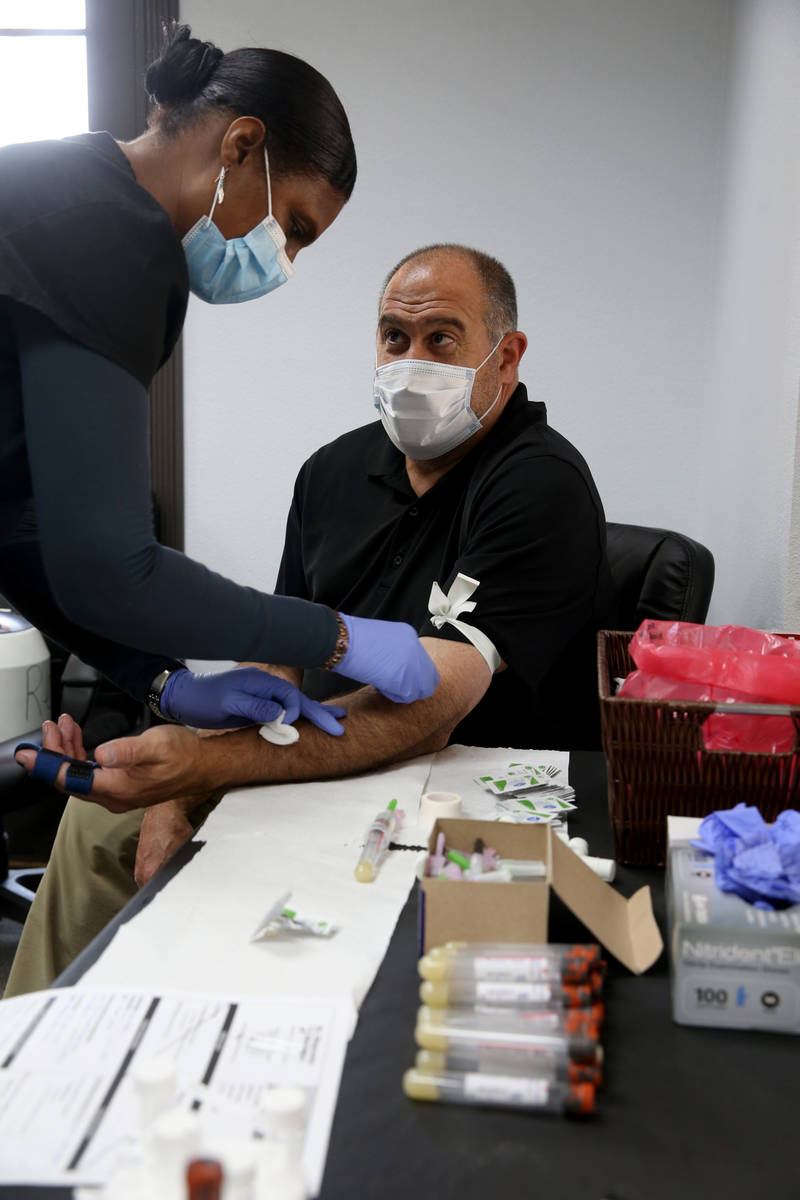
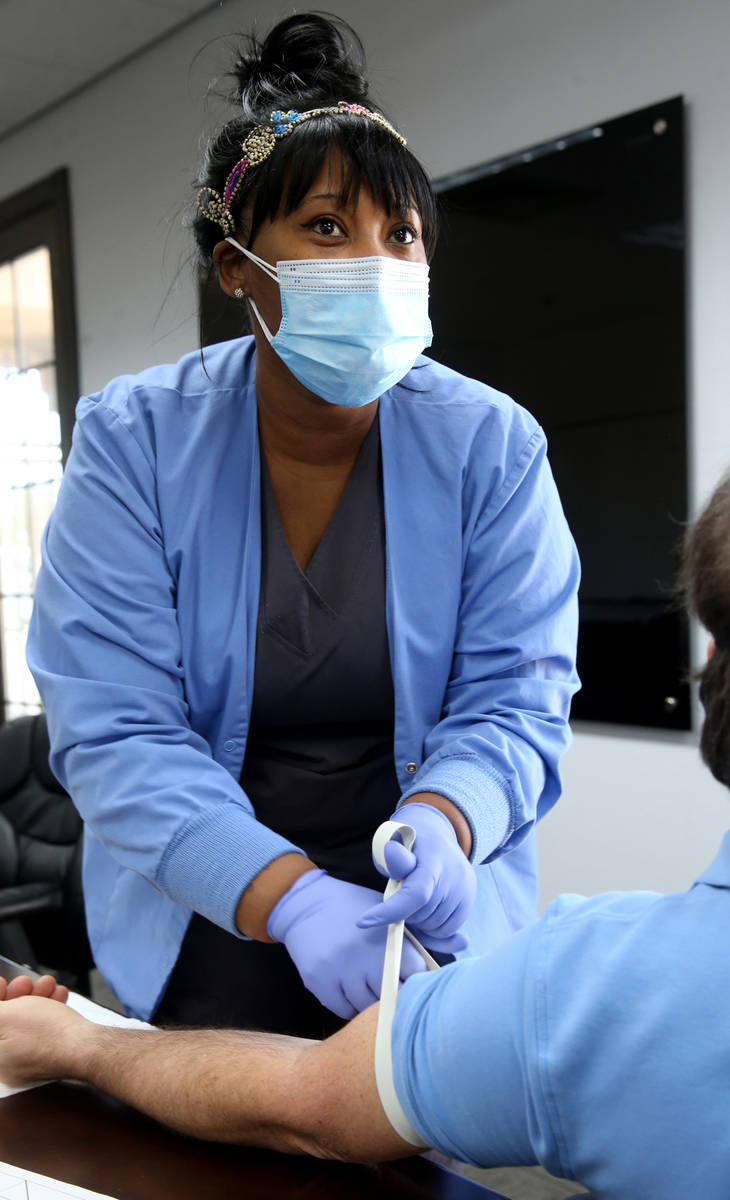
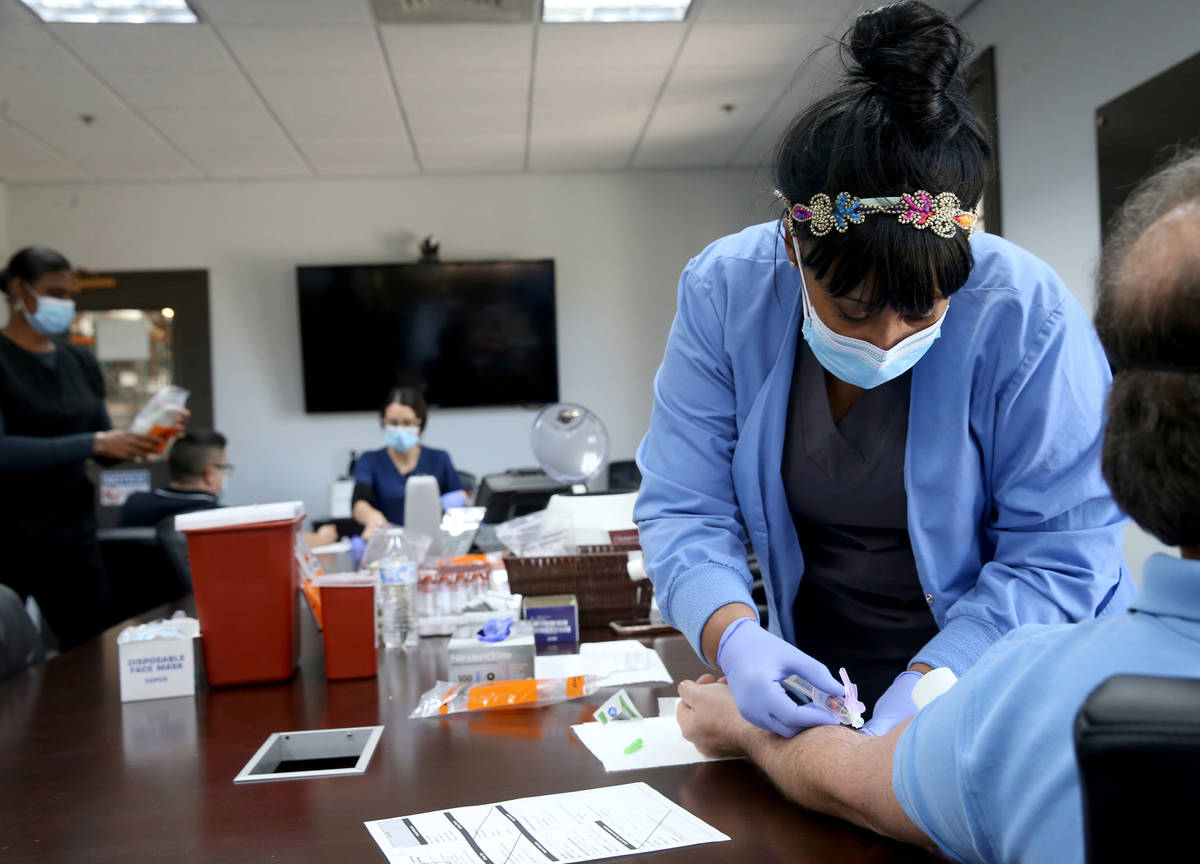
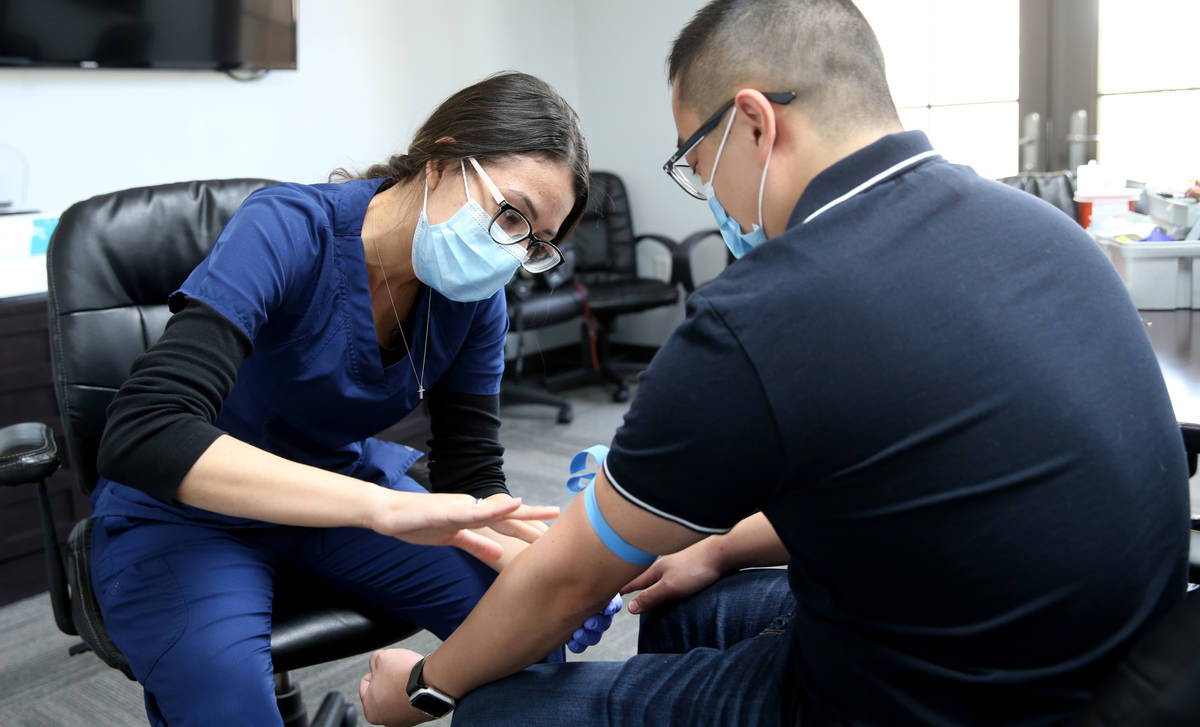
At a time when antibody testing could be key to reopening society, a manufacturer of dietary supplements is providing free tests to its entire U.S. workforce, including 90 employees at its Henderson plant.
In providing the antibody testing, Designs for Health hopes to learn what percentage of its workforce might have immunity to the virus that causes COVID-19, while giving employees “some comfort and peace of mind,” said Dr. David Brady, the company’s chief medical officer.
“We’re doing this because our employees want this,” Brady said. “They want to know.”
The unusual step comes at a time when public health authorities in Nevada and across the country are ramping up to conduct antibody testing to better understand the behavior of the virus and guide public policy.
Antibodies are proteins produced by the immune system to neutralize a pathogen such as a virus — in this case, the new coronavirus. Once an individual has developed certain antibodies, that person is expected to have some level of immunity to the disease. With the new coronavirus, however, it’s unknown to what extent these antibodies provide immunity or for how long, though authorities have said that a six-month window of protection is a reasonable assumption.
Testing offered Thursday
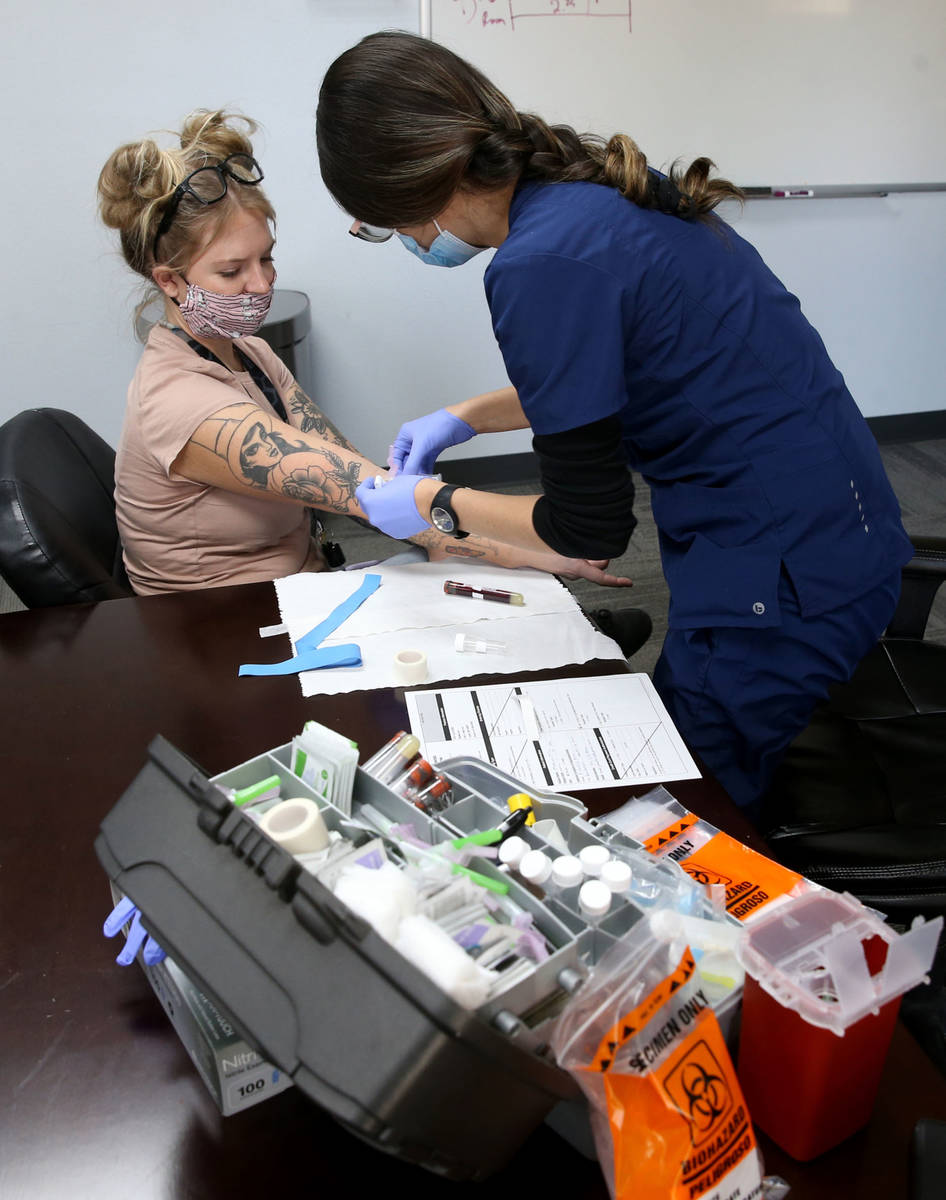
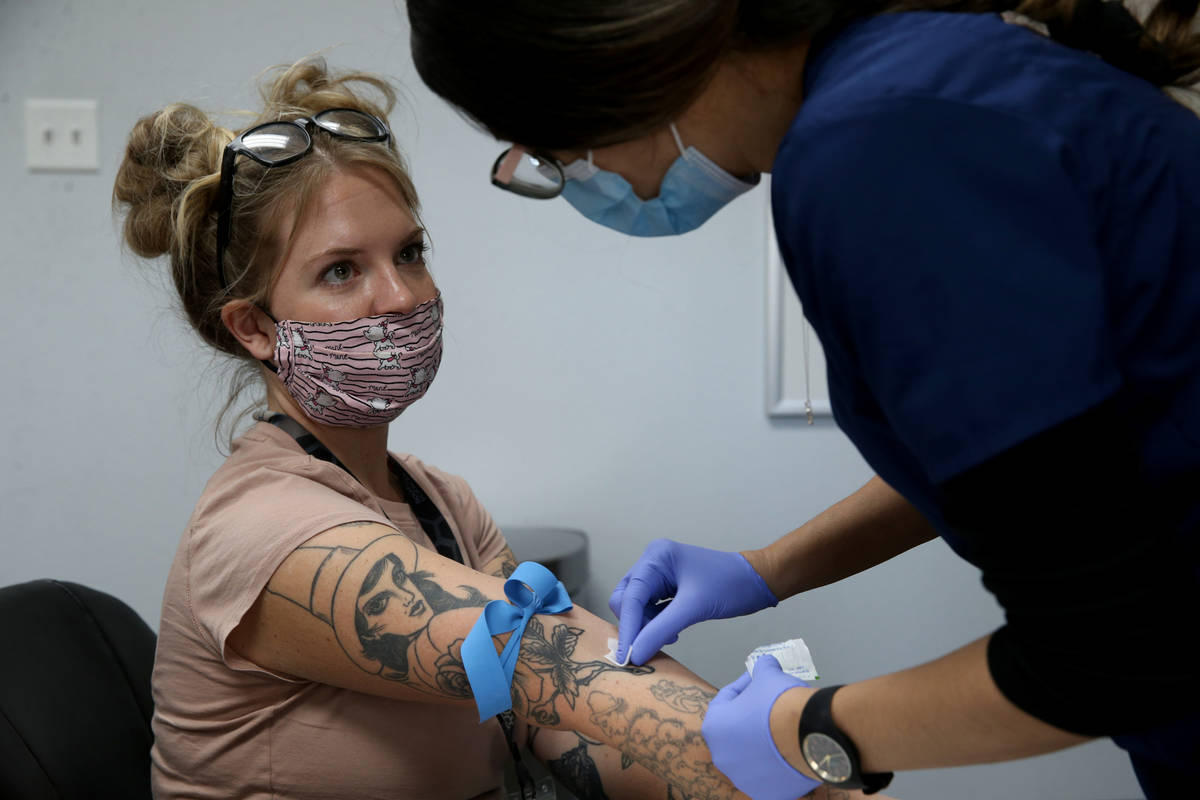
The company offered antibody testing to its 400 employees at four locations around the country on Thursday, including to its 90 employees in the Las Vegas Valley. Testing was strictly voluntary, Brady said. The company does not see the individual test results of employees but will receive aggregate data on percentages at each location who tested positive.
“We’re running the plants 24/7 in all our locations,” Brady said of the company, which makes nutritional supplements for health care professionals and their patients. “We need a very healthy and intact workforce.”
The company has been deemed an essential business, and its employees continue to work in functions such as manufacturing, shipping and receiving across the country.
More than 100 employees were tested earlier this week, though Brady said no results were yet available.
The test by Diagnostic Solutions Laboratory checks for two types of antibodies — one that develops at the onset of an infection and another that develops as a person recovers.
Employees are encouraged to tell their managers if testing shows they are in the early stages of infection so that they can take paid time off and seek appropriate medical care.
Brady didn’t envision any immediate company procedural changes from the testing,
“We’re not trying to use it for some strategic reason with our employees at this point,” he said.
But he believes that antibody testing will need to be done on a broader basis to guide companies’ back-to work strategies.
“We’re going to have to do this on a company by company, state by state, region by region basis,” he said.
Uses for testing
The Nevada State Public Health Lab in Reno is ramping up to conduct antibody testing in the coming weeks, but it remains unclear how the tests will be conducted.
“Data from antibody tests will help state leaders make informed business and public health decisions,” according to a news release from Gov. Steve Sisolak’s Nevada Health Response task force.
Mark Pandori, the lab’s director, said it would be up to health districts and clinicians to determine who gets tested, where patients can get tested and how the tests will be administered.
A spokeswoman for the Nevada Department of Health and Human Services said Thursday that a plan for antibody testing was being developed with the state lab.
Brian Labus, who serves on the governor’s COVID-19 medical advisory team, said that he expects antibody testing would be used to better understand patterns of disease transmission. For instance, with a disease outbreak in a nursing home, antibody testing could fill in details such as how far the disease spread through people who displayed no symptoms of illness.
He’s not persuaded, however, that antibody testing holds the key to reopening society.
“I don’t think it’s going to give us the information we need for reopening,” he said.
If antibody testing were to show that high percentages of people had developed some immunity, that could drive some of the reopening decision-making, he added.
Studies in Santa Clara County and Los Angeles County indicated that less than 5 percent of people in those counties had the antibodies. However, on Thursday, preliminary results of random testing of 3,000 residents of New York City showed that 20 percent — or one in five — had antibodies. The studies all indicate that the number of infected people vastly exceeds the number of confirmed cases.
Advantages, potential drawbacks
People can benefit personally from antibody testing. Labus noted that many people with symptoms were unable to be tested because of limited COVID-19 testing and want to know if they had the disease.
With knowledge of immunity status, a person is able to make more informed decisions related to behavior and risk, Labus said. A person who tests positive for antibodies might take on a household’s grocery shopping in place of more vulnerable members.
He cautioned, though, that the market has been flooded with antibody tests of varying quality under emergency use authorization by the U.S. Food and Drug Administration, which loosened regulation in the face of a public health emergency. Early attempts to provide drive-thru antibody testing in the Las Vegas Valley were suspended by state authorities last week over regulatory issues.
Brady believes that enthusiasm for antibody testing may have waned because authorities “don’t know what to do with a partitioned population,” where society is divided between people who are susceptible to the virus and those with some level of immunity because of exposure.
There may be concern that those with antibodies think they’re “bulletproof and they don’t need to pay attention to all this social distancing stuff,” he said.
“It brings up this conundrum,” Brady said, adding, “I don’t know what the answers are.”
Contact Mary Hynes at mhynes@reviewjournal.com or 702-383-0336. Follow @MaryHynes1 on Twitter.