Cleveland Clinic seeks treatment for Alzheimer’s
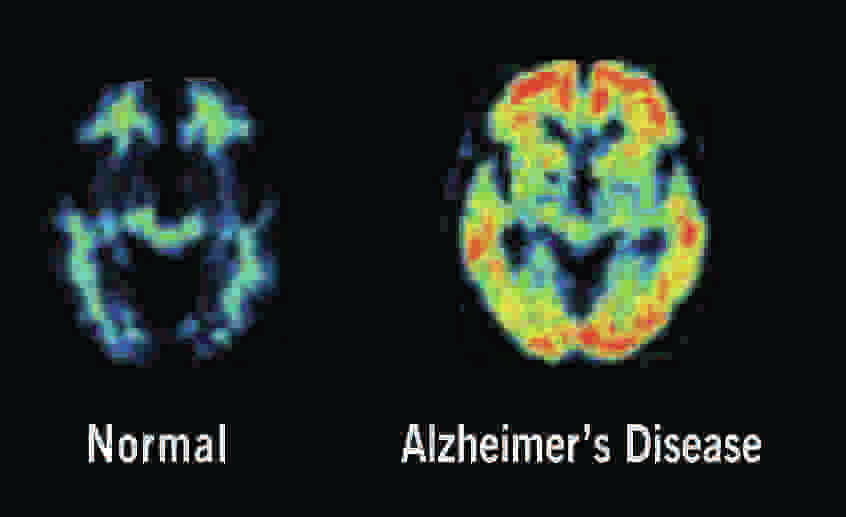
Editor’s note: While Robin Leach is on vacation, Dr. Jeff Cummings, head of the Cleveland Clinic Lou Ruvo Center for Brain Health, will be the guest column today. He will give us an update on the center’s work in the battle against Alzheimer’s and other brain degenerative diseases.
……
Currently, there is a gray tsunami lurking on the horizon, posing an eminent threat socially and economically. The tsunami will make landfall by 2030, when it’s estimated that nearly 75 million people worldwide will have Alzheimer’s disease (AD), with the cost of caring for these individuals reaching $1 trillion.
This is a financial burden that we cannot bear, which is why the Obama Administration, as well as several member nations attending the 2013 G8 Dementia Summit, established a goal of developing a meaningful treatment for AD by 2025. Since then, researchers worldwide have been racing to develop a cure or treatment.
Time is ticking and in an effort to better understand where we are at in the AD drug development process, I, along with fellow researchers at Cleveland Clinic Lou Ruvo Center for Brain Health as well as colleagues from Touro University and Global Alzheimer’s Platform, have begun a yearly analysis of the AD drug pipeline.
We recently concluded our second assessment and published results in the paper called “Alzheimer’s Disease Drug Development: Pipeline 2017” in the Journal of Alzheimer’s & Dementia: Transitional Research & Clinical Trials Interventions.
The paper sheds light on the immediate challenges of AD drug development and the urgent need to increase the number of agents entering the pipeline to accelerate the drug-testing process.
Using a comprehensive analysis of ClinicalTrials.Gov, where all clinical trials are registered (by law), we examined all clinical trials from 2016 to 2017 and found that the number of agents in the pipeline is small — 105. In addition, compared to the 2016 pipeline, there are only eight new agents in Phase 1, illustrating a desperately slow period in AD drug development.
To meet our 2025 goal, an agent must currently be in the second of three phases of drug development. The third phase leads to Food and Drug Administration review and possible approval. Given the state of the pipeline, the approval of one or two drugs is plausible, but the goal of developing an effective treatment or prevention for many of the patients with AD is in jeopardy.
In addition to high drug failure rates, we also uncovered obstacles that inhibit drug development, which include slow clinical trial recruitment and drug testing as well as a lack of sufficient funding. Currently, the National Institute of Health spends an annual $6 billion on cancer research, more than $4 billion in heart disease research, $3 billion on HIV/AIDS research and less than $2 billion on AD research.
Cleveland Clinic believes we must address these inhibiting factors in order to increase the success rate and accelerate the development of new therapies. Our paper serves as a call to action and, as a leader in research, we are ready to help orchestrate the changes needed to enhance the drug-testing process.
While there are countless ways to improve the AD pipeline, we have identified several areas we believe can significantly advance drug development. Repurposing drugs already approved by the FDA to treat other diseases can help speed up the drug development timeline and increasing investments in basic research can aid in identifying new therapies.
Slow recruitment of participants is the most expensive part of clinical trials and arguably, the greatest challenge in drug development. A national effort is needed to address this issue as a staggering 54,000 participants are needed to complete the current list of AD trials. The Global Alzheimer’s Platform — a nationwide program in which Cleveland Clinic takes a leading role — was created for this very reason. One way to expedite recruitment is to utilize biomarkers such as brain imaging to identify qualified clinical trial candidates.
The need is great, the challenges are many, the rewards are high — this is the status of the AD pipeline. As a scientist, I remain hopeful that an effective treatment is in sight because there has never been a greater, more coordinated effort to develop a cure. Amazing work takes place daily at the Lou Ruvo Center for Brain Health and we are at the forefront of ground-breaking research that will impact the world.












