Second doctor tells medical board he reused needle guides in biopsies
When Dr. Lawrence Newman learned that the FDA and the Nevada State Medical Board sanctioned Dr. Michael Kaplan in early March for reusing single-use medical devices during prostate biopsy procedures, he reported himself to the board and began notifying his patients, according to Doug Cooper, the board’s executive director.
"He saw what happened to Kaplan and realized he was doing the same thing," Cooper said. "He’s already contacted the patients involved and verified he is going to pay for any testing. But his case obviously remains under investigation."
Newman, whose office in Las Vegas is listed at 5380 South Rainbow Blvd., incorrectly believed he could sterilize single-use plastic endocavity needle guides, Cooper said, adding that the physician did 150 prostate biopsies over a three-year period during which single-use needle guides were reused.
On the Internet, Newman, a urologist, also lists a California office.
A source close to the state’s infection control efforts said Newman, like Kaplan, told authorities that a vendor had said the plastic needle guides could be used more than once.
A vendor told Kaplan, whose medical license has been suspended, that the plastic needle guides could be used three to five times, according to an ad written for the Review-Journal by Kaplan attorney Dominic Gentile.
Authorities said Kaplan, against whom the board filed a more detailed formal complaint Wednesday, stopped using single-use endocavity needle guides only when they became "too bloody."
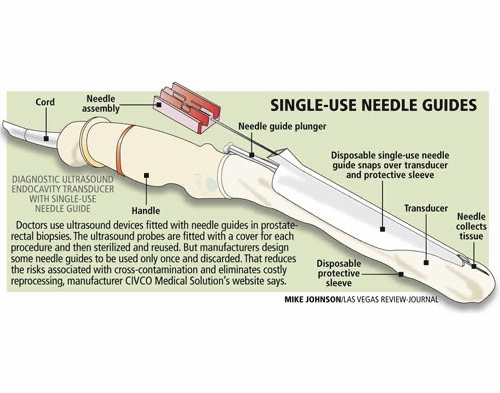
The single-use needle guides, plastic sheaths through which needles are directed to obtain biopsy material, regularly come in contact with blood and body fluids, which could be passed to another patient if reused.
Gentile said Wednesday that a Providian Medical Equipment vendor — who was distributing needle guides made by CIVCO Medical Solutions — admitted to one of his law firm investigators that he told Kaplan that the plastic needle guides could be used more than once.
"He (the Providian vendor) said he had been told that they could be reused by someone at the manufacturer (CIVCO)," Gentile said. "I was surprised that he told the truth, given the potential liability."
Gentile said he expects that many more doctors than Kaplan and Newman have reused the single-use needle guides in Southern Nevada: "I have had five or six doctors call me and confirm that’s what they were told, too. "
Gentile now believes, however, that the problem is a national one, not just one affecting Nevada. He said Providian distributes CIVCO equipment throughout the nation.
"My best guess is we’re talking about thousands, maybe tens of thousands of people, who have had biopsies done with reused single-use devices," the attorney said. "This isn’t an attempt to justify what was done. This shouldn’t have happened. It’s an explanation."
Attempts to reach representatives at both Ohio-based Providian and Iowa-based CIVCO were unsuccessful.
Newman also was unavailable for comment Wednesday.
The Southern Nevada Health District notified 101 patients of Kaplan, who began reusing single-use devices in December and stopped in early March, that they had to get tested for HIV and hepatitis.
The formal complaint against Kaplan issued Wednesday says 115 procedures were done. Medical officials could not be contacted late Wednesday to explain the discrepancy.
To date, the health district has no reports of any of Kaplan’s patients contracting a blood-borne disease as a result of his breach of infection control.
Jennifer Sizemore, a spokeswoman for the health district, said the district will not send out letters to Newman’s patients because he already has done so. She said he sent out letters to all his patients, informing them that if they had a biopsy procedure, they should get tested for HIV and hepatitis now and again in six months.
Physicians should always remember that vendors "are trying to sell you something," said Dr. Dale Carrison, chief of staff and head of the emergency room at University Medical Center.
"They’re businessmen, not doctors," he said "If you’re well trained, you’ll remember that."
Carrison said it is conceivable that doctors become too close to vendors.
"You become friendly and stop being skeptical," he said. "Selling you something is what they do for a living. You have to remember you’re not talking to another physician."
If a vendor says a single-use item can be used more than once, the physician should check with the Food and Drug Administration, Carrison said.
"If the FDA says you can, then you can," he said.
There are 100 types of single-use medical devices that can be reprocessed and used again for a single use, but the plastic endocavity needle guide reused by Kaplan and Newman is not one of them.
And those single-use items that can be reprocessed for another single use cannot be reprocessed in a physician’s office. They can only be reprocessed by an FDA-approved facility. Neither Kaplan nor Newman owns such a facility.
Cooper, of the state medical board, said it is unknown at this time whether Newman’s license will be suspended. The board’s website lists no disciplinary actions against Newman.
"You have to remember that the facts of the case against Newman are different than Kaplan’s," Cooper said. "The way it came to our attention is different. He self reported to the board and had a list of patients ready to notify."
In the formal complaint filed Wednesday against Kaplan, which charges him with malpractice and the continued failure to exercise an ordinary standard of care, the physician is said to have instructed his staff to reuse needle guides, even though a reference guide shipped with the devices did not include any instructions for cleaning them, only directions for "single-use components to be disposed of as infectious waste."
"After procedures using the disposable needle guides, medical assistants would attempt to clean and disinfect the needle guides by running them under water, attempt to scrape out any remaining tissue or blood without using a brush and would then soak them in Cidex solution and allow them to dry," the complaint states. "Often bits of tissue or blood would remain after attempted cleaning and disinfecting. Guides were disposed of after becoming too bloody."
Contact reporter Paul Harasim at pharasim@review journal.com or 702-387-2908.