Clinical trials give cancer patients hope, time
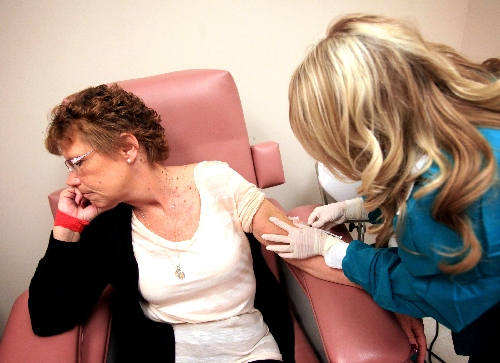
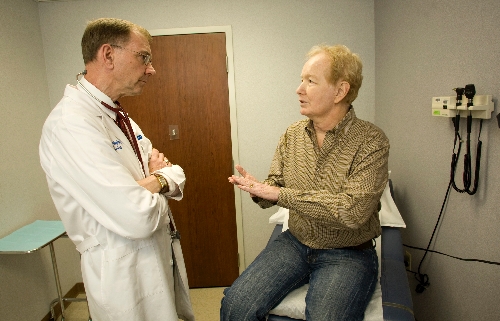
Linda Shreve stopped by her doctor’s office Monday to get a shot. John Quinn stopped by his to be examined for upper respiratory problems.
These visits would seem to be part of the routine medical care that goes on every day in the Las Vegas Valley.
Yet they were anything but. Both Shreve and Quinn are involved in nationwide clinical trials for drugs that could prolong life for those with cancer.
She is fighting lung cancer. He is dealing with cancer of the prostate, a small walnut-sized structure that makes up part of a man’s reproductive system.
The drug trial for 57-year-old Shreve involves Lucanix, a novel cancer vaccine being tested in patients with non-small cell lung cancer who have completed several rounds of chemotherapy. If it works, it will improve patient survival by programming the body to fight off any remaining cancer cells.
Quinn, 63, is taking daily in pill form XL184, an experimental drug showing effectiveness in treating tumors that have spread to the bone, a major cause of death and disability for men with prostate cancer. He is fast running out of options for survival.
He and his wife, Roxane, were worried Monday that a side effect of the experimental drug could be breathing difficulties. But after examining Quinn, Dr. Nicholas Vogelzang, who was coughing himself, assured Quinn that the XL184 wasn’t behind his wheezing, but rather “a bug you picked up that is going around.”
Quinn, who has tried several drugs without success to control the spread of his cancer, was pleased at the news.
“I don’t want this drug to end,” he said. “It keeps me out of pain.”
Vogelzang, a world-renowned cancer researcher treating Quinn and other Las Vegas men with XL184 at the Comprehensive Cancer Centers of Nevada site off Eastern Avenue near Desert Inn Road, has heard similar comments during drug trials.
“Clinical drug trials are important,” he said. “They allow people to receive state-of-the-art cancer care in Nevada, the newest treatments available.”
In 2010, Comprehensive Cancer Centers had 86 open clinical trials, using research affiliations with UCLA’s Jonsson Comprehensive Cancer Center/Translational Oncology Research International and US Oncology to bring drug trials to Las Vegas.
The Nevada Cancer Institute, where researchers try to develop new cancer fighting drugs, had 70 clinical drug trials in 2010.
“Despite our best efforts over several decades in this country, we have not come up with cures for this disease,” said Dr. John Ruckdeschel, CEO of the Nevada Cancer Institute. “Clinical research must continue until we get the right therapies.”
Shreve gets an injection as part of her drug trial once a month at the Comprehensive Cancer Centers site adjacent to the St. Rose Dominican Hospital, Siena campus. Under the care of oncologist Dr. Rupesh Parikh, she is fully aware that she may not be receiving Lucanix, but a placebo instead. The study must meet strict scientific standards if the drug is to be approved by the Food and Drug Administration.
“If this is something I can do that can help in the fight against cancer, I want to be part of it. And I hate shots,” Shreve said after a nurse gave her an injection that left her grimacing.
So far, the only side effect of the shots Shreve has had over the last few months is a temporary lump on the upper part of her arm.
Early results have been encouraging for the Lucanix trial that began in 2008. More than 60 percent of the people who had the vaccine lived at least another year following chemotherapy for lung cancer, and 40 percent lived two years. Normally, only 30 percent survive past the first year after receiving chemo.
Lung cancer kills some 160,000 Americans a year, more than breast cancer, colon cancer and prostate cancer combined. About 85 percent of people with lung cancer will die within five years of being diagnosed, because the disease usually isn’t caught until its late stages and there are few effective treatments, according to the American Cancer Society.
Before she signed up for the clinical trial, Shreve already had received good news. The regimen of chemotherapy, radiation and surgery she endured after her 2009 lung cancer diagnosis had left her without signs of cancer.
“I’ve just never believed I would die from it,” said Shreve, an administrator with Richmond American Homes builders in Las Vegas. “I’ve just been very positive. I’m praying I remain cancer free. God knows I am.”
Though a former cigarette smoker, she believes her cancer — and several different cancer types suffered by other members of her family — is the result of growing up in southwest Utah, where radioactive fallout drifted from above-ground nuclear bomb tests in the 1950s and 1960s at the Nevada Test Site.
She is filing paperwork for compensation up to $50,000 that the federal government has made available to victims of the tests, who are known as “downwinders.”
While Parikh is hopeful that Shreve’s lung cancer will remain in remission, he cautioned that “hasn’t been the history of the disease. … We’ve found that it comes back.”
That’s why Lucanix, or a drug like it, is so necessary, Parikh said.
“Obviously, we want to maintain Linda Shreve’s great response to the treatment she’s had,” he said, adding that three local women are part of the Lucanix trial.
Quinn, a movie and TV producer, was diagnosed with prostate cancer in 2002 while living in Southern California. A routine PSA test, which measures the amount of prostate specific antigen in the blood, came back high.
The disease is the third most common cause of death from cancer in men of all ages and is the most common cause of death from cancer in men over age 75. The cause of the cancer is unknown.
The overall five-year survival rate for prostate cancer if it remains localized is almost 100 percent, with surgical and radiation treatment very successful if the disease, which is generally slow-growing, is caught early. If the cancer spreads from the region, however, the survival rate at 5 years falls to 31 percent.
Quinn worries that his attempt at holistic treatments when he first was diagnosed, rather than the conventional surgery or radiation, may have not been wise.
“I just don’t know. I do think you have to combine the holistic, things like proper diet, with conventional medicine.”
The father of two grown children, Quinn thought he had beat the disease through drug treatments. But it came back in 2005, and he started seeing Vogelzang, then director of the Nevada Cancer Institute.
A variety of hormonal treatments, new drugs and chemotherapy would work for a while, but then the cancer would progress. Pain that “felt like toothaches” spread throughout his body.
The chemotherapy Quinn had prior to the XL184 not only was uncomfortable, it nearly killed him when it caused blood clots, including one that blocked his pulmonary artery.
“I had to be rushed to the hospital for that,” he said.
Still, even with the chemo producing severe mouth sores, numbness in his extremities, a lack of appetite and extreme fatigue, he kept working on video equipment he has in his Las Vegas home, producing the cable TV series “Sin City Diaries” for Cinemax.
Last June, Vogelzang had hoped Quinn could receive the newly FDA-approved Provenge, a novel therapy for fighting prostate cancer that became the first cell-based immune regimen approved by the government. It involves taking a patient’s own white blood cells, which fight infection, and mixing them with a bioengineered molecule similar to one found on nearly all prostate cancer cells.
The genetically bolstered cells, which are infused into a patient on three separate occasions over about a monthlong period, would help the patient’s immune system identify the cancer cells in order to attack them.
“But it turned out I didn’t have enough white blood cells to do it,” Quinn said. “My other treatments robbed me of them. That really depressed me. I basically thought I was out of options.”
But then Vogelzang came upon the new XL184 clinical trial. Early test results of the drug showed that 19 of 20 patients with evident bone metastasis achieved either complete or partial resolution of their lesions on bone scans.
“I slept a lot at first when I took the drug, but all my pain went away in three days and bone scans showed a reduction in cancer,” Quinn said. “Dr. V. reduced the dosage and being overly tired hasn’t been a problem.”
The side effects of the drug have been minor: a slight metallic taste in the mouth, an accentuated whiteness to his skin, and diarrhea.
“The right amount of Immodium takes care of that,” Quinn said, laughing.
Under the protocols set for the XL184 drug trial, each participant was to take the drug for just 12 weeks. Some who did well were to be taken off it, Quinn said. “But then the pain came right back in my body. My life became hell.”
Bone scans showed the cancer was growing again.
If that happened, researchers were allowed to put a clinical trial participant back on the drug. Vogelzang did so immediately.
Quinn fears that, like other drugs he has tried, XL184 eventually will stop working.
“I’ve always known it’s not a cure, but I have had five wonderful months of no pain,” he said.
“Even if it stopped working tomorrow and I ran out of alternatives and died, I still would have to be happy that I was able to live a life without pain for a while, that I could go places, enjoy my family.”
Vogelzang doesn’t want Quinn to give up hope.
“It looks like your white blood cells are coming back,” he told Quinn as he looked at test results. “I think we’ll be able to get you on Provenge, after all.”
Contact reporter Paul Harasim at
pharasim@reviewjournal.com or 702-387-2908.
If you are interested in participating in clinical cancer trials, contact:
• Comprehensive Cancer Centers of Nevada at 702-952-3400, ext. 5363
• Nevada Cancer Institute at 702-822-LIFE (822-5433)












