Cancer patients find new hope
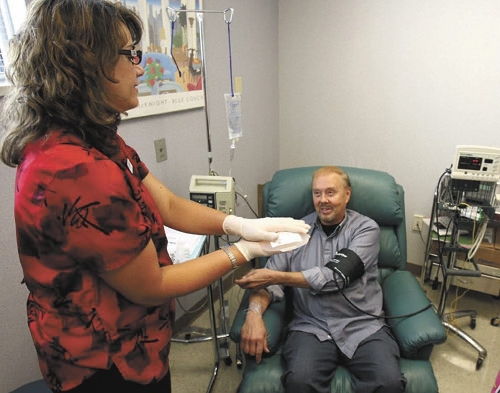
The prescription medication made from his own immune cells flowed into the arm of Jim Phillips as he sat inside a Comprehensive Cancer Centers of Nevada transfusion room on Eastern Avenue.
A huge smile was on the face of the retired 69-year-old United Airlines captain Friday afternoon as he talked about becoming the first Nevadan to receive FDA-approved Provenge, a novel technique for fighting prostate cancer that in April became the first cell-based immune therapy approved by the government.
“I hope this does what it’s supposed to do,” said Phillips, who will be one of only 2,000 men across the country in the next year to receive the treatment.
It involves taking a patient’s own white blood cells, which fight infection, and mixing them with a bioengineered molecule similar to one found on nearly all prostate cancer cells.
“This treatment certainly seems to make sense,” Phillips said.
The genetically bolstered cells, which are infused into a patient on three separate occasions over about a month-long period, should help the patient’s immune system identify the cancer cells in order to attack them.
The new therapy for men with advanced prostate cancer is the culmination of decades of research into harnessing the immune system to fight tumors, an approach that many researchers believe can also be used in treating other diseases, such as HIV.
A few Las Vegans had access to the treatment regimen four years ago when the largest clinical trial of Provenge was conducted at 50 centers across the United States and included a half dozen patients at the Nevada Cancer Institute.
They were among 512 seriously ill men who were studied to see whether the drug should be put on the market.
The study found that those who received Provenge had a median survival of 25.8 months after treatment, while those who got a placebo lived 21.7 months.
After three years, 32 percent who received Provenge were still alive, compared to 23 percent who got the placebo.
Around 192,000 men are diagnosed with prostate cancer each year, and more than 27,000 die from it, according to the American Cancer Society.
That the approved therapy is now available in Nevada is largely due to the efforts of Dr. Nicholas Vogelzang, the former director of the Nevada Cancer Institute and current developmental therapeutics medical director for U.S. Oncology. The medical consortium provides care to thousands of cancer patients nationwide and does business locally as Comprehensive Cancer Centers.
“We worked very hard to get a clinical trial at the cancer institute in 2006-2007,” said Vogelzang, an internationally renowned researcher in oncology still based in Las Vegas. “It’s exciting that patients in Las Vegas now have access to this treatment.”
Because of a shortage of the drug therapy, the manufacturer, Dendreon Corporation of Seattle, is only making it accessible this year to centers and physicians who participated in the clinical trials.
Vogelzang said one of the patients from Las Vegas who was involved in the clinical trial at the Nevada Cancer Institute has been alive for four years with no need for chemotherapy.
In the not too distant future, Vogelzang said, it is very possible that prostate cancer patients will get the drug when they are not so ill and have a better chance of long-term survival from the therapy.
To date, Vogelzang said, the FDA has only approved studies done with Provenge involving patients whose cancer has spread outside the prostate and no longer responds to hormone therapy.
The fact that the Nevada Cancer Institute was one of 50 centers in the country to participate in the trials that led to FDA approval of Provenge must not be forgotten, said Dr. John Ruckdeschel, the institute’s current CEO and director.
“That’s why we’re here,” he said of the research center that opened five years ago. “Without the institute, the treatment wouldn’t be available.”
Ruckdeschel even took out a front-page advertisement for the cancer institute in the Review-Journal that he hopes will remind the public of the institute’s mission at the same time it attracts patients there for the Provenge treatment.
“We’re getting calls from all over,” said Dr. Stephen Patterson who heads up the cancer institute’s Provenge treatment. “This new treatment is creating a lot of buzz.”
Vogelzang has several patients lined up for the treatment at Comprehensive Cancer Centers of Nevada, including Lynn Leany, a 69-year-old steel contractor.
Leany sat in his office recently off Tropicana Avenue and Rainbow Boulevard and talked of the surgery and drug treatments he has undergone since he was diagnosed in 1994. He looks forward to receiving Provenge at the end of June.
“I’m kind of willing to try almost anything, but this sounds like the right thing to do,” he said, laughing. “I’ve got too much to live for to die. It’s like my grandmother said: ‘I know what’s here. I’m not sure what’s on the other side.’ ”
Provenge often is referred to as a vaccine, though it doesn’t prevent cancer, unlike a measles or polio shot.
The main side effects for Provenge in clinical trials were flu-like symptoms, such as fever and chills. By comparison, chemotherapy is often toxic, leading to infection, pain and muscle, bone and hair loss.
When the FDA declined to approve the drug three years ago, some prostate cancer patients and investors protested.
Two officials who opposed approval of the drug received death threats.
“I can understand that happening,” Leany said. “You’re dealing with people’s lives.”
The series of three Provenge infusions or shots will cost $93,000, a price Dendreon officials defend by saying it is in line with those of other cancer-fighting drugs that increase survival rates by months.
The officials have said the company had spent about $1 billion developing Provenge. On the day Provenge was approved by the FDA, Dendreon’s stock rose to $50.18, more than double its level a year ago; company officials predicted sales of the treatment regimen will reach $1 billion annually within two or three years.
Advanced prostate cancer, which Provenge treats, is found mostly in men over 65 years of age, when individuals are covered by Medicare.
Ruckdeschel said government officials say taxpayer supported health insurance will cover the cost of the drug, and he said cancer centers will not profit heavily from the labor intensive treatment.
He noted that only certain blood collection centers have been approved to engage in a process called apheresis, where patients are attached to what looks like a dialysis machine. But instead of cleansing the blood, it takes out the white blood cells and gives the patients back their red blood cells.
Last week Phillips had to travel to Salt Lake City for the treatment. His white blood cells were then sent to the drug manufacturer, where the bioengineering takes place.
The cell product that was left was then shipped to Vogelzang in Las Vegas so Phillips could be infused.
“It took about three or four hours in Salt Lake, but it wasn’t bad at all,” said Phillips, who set three world speed records flying the B-747-400 and has been honored at the Smithsonian.
Vogelzang said it is possible that in the future a blood collection center will be approved in Las Vegas.
First diagnosed with prostate cancer in 1996, Phillips thought he had it beat after receiving proton therapy, a precise form of radiation treatment, at Loma Linda University Medical Center in California. But in 2003 he began to have problems again.
“This has been quite a ride emotionally,” he said. “I’ve tried different treatments that haven’t worked, but Dr. Vogelzang won’t give up. I appreciate that. We’re sort of going through this together. Neither one of us has any interest in dying.”
Contact reporter Paul Harasim at pharasim@review journal.com or 702-387-2908.